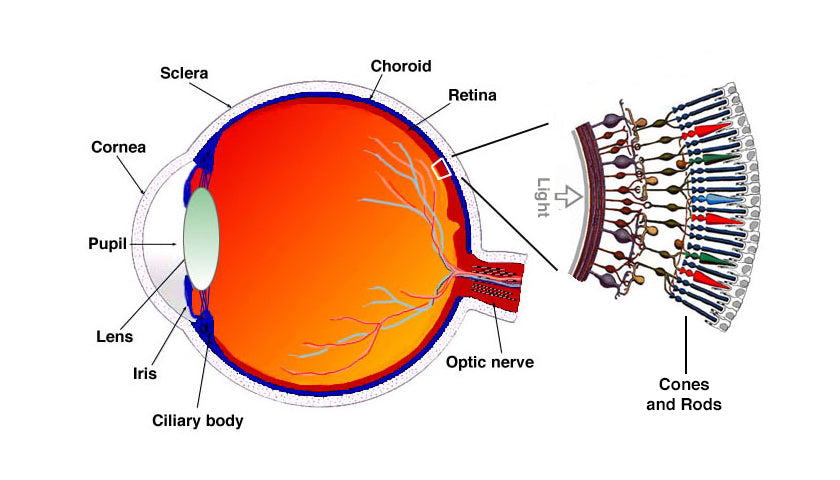
The molecule that takes part in the initial step in the vision process, rhodopsin, has two components called 11-cis retinal and opsin. Retinal is a light-sensitive derivative of vitamin A, and opsin is a protein molecule.

Rhodopsin is found in the rod cells of the retina, which lines in the internal wall of the eye. 11-cis retinal is a powerful absorber of light because it is a polyene; its 6 alternating single and double bonds make up a long unsaturated electron network. When no light is present, the 11-cis retinal molecule is found in a "bent configuration" (fig A), and as such it is attached to the opsin molecule in a stable arrangement. When light strikes the retina, within 200 femtoseconds, after the retinal molecule absorbs a photon into one of the pi bonds found between the eleventh and twelfth carbon atoms, the 11-cis retinal is transformed into the all-trans retinal (fig B) in a straightened configuration.[1] Retinal molecule - straightens in response to a photon γ (light), of the correct wavelength Retinal molecule - straightens in response to a photon γ (light), of the correct wavelength
The all-trans retinal configuration, subsequently, does not fit into the binding site of the opsin molecule; as a result, upon isomerization, the trans isomer separates from the protein, which triggers a G protein signaling pathway' including transducin, that results in the generation of an electrical impulse, which is transmitted through the optic nerve to the brain for processing. It takes a minimum of five photons to trigger a nerve impulse.[2] In the absence of light, enzymes mediate the isomerization of all-trans back to the 11-cis configuration, and rhodopsin is regenerated by a new formation of a Schiff base linkage, which actuates the binding of the cis isomer to opsin. This is the basic mechanism of the vision cycle.
All-trans-retinal is also an essential component of type I, or microbial, opsins such as bacteriorhodopsin, channel rhodopsin, and halorhodopsin. In these molecules, light causes the all-trans-retinal to become 13-cis retinal, which then cycles back to all-trans-retinal in the dark state.